implantable rfid chip in health care bill Since 1998, RFID chips have also been implanted in humans. This practice is little studied but appears to be increasing; rice-sized implants are implanted by hobbyists and even offered by some employers for uses ranging . Show product RATECARDS™ - Google Review Card Premium Black. Before RATECARDS™ staff had to ask customers to leave reviews or follow your company page, or even worse, scan a QR code. Now your customers just has .Our Tap review card is the easiest way to amplify your online presence with 5-star reviews. .
0 · Will 'Obamacare' Legislation Implant U.S. Residents with
1 · What Are the Benefits and Risks of Fitting Patients with
2 · What Are the Benefits and Risks of Fitting Patients with
3 · The microchip implants that let you pay with your
4 · On Emerging Technology: What to Know When Your
5 · Must Citizens Who Want to Receive Government Benefits Agree
6 · Ethical implications of implantable radiofrequency identification
7 · Did Congress Pass a Bill Allowing the Government to Microchip
8 · Are You Ready for a Medical RFID Implant?
9 · AMA Code of Medical Ethics’ Opinions Related to Implantable
NFL playoffs probabilities, NFL postseason standings for every team entering NFL Week 11 with the Cardinals, Eagles, Lions, Chargers' chances rising.
Claim: Health care legislation requires that U.S. residents be implanted with RFID microchips.The callers' anxiety stemmed from an article on a website called National Report, .
Claim: H.R. 4919, passed on 8 December 2016, allows the microchipping of "mentally disabled" citizens such as patients with autism and Alzheimer's disease.
Since 1998, RFID chips have also been implanted in humans. This practice is little studied but appears to be increasing; rice-sized implants are implanted by hobbyists and even offered by some employers for uses ranging . Many critics, including state legislators working to pass bills that would restrict RFID implants, are fearful that the metal components and . The implant needs to be within the electromagnetic field of a compatible RFID [or NFC] reader. Only when there is a magnetic coupling .
Many US patients will have an implantable device during their lives. The AMA Code of Medical Ethics offers guidance for weighing need for patient-subjects’ safety against health care sector demand for innovation.
Patients must trust that RFID devices will not be implanted or removed without their prior consent. When seeking patients' consent to implant an RFID device, physicians .
The implanted RFID devices enable patients to establish health care identities and become the stewards of their own data. The patient can assemble a reconciled medication list, a complete .This article reviews the use of implantable radiofrequency identification (RFID) tags in humans, focusing on the VeriChip (VeriChip Corporation, Delray Beach, FL) and the associated .
Claim: U.S. citizens who receive government benefits will soon be required to have microchips surgically implanted in them.Claim: Health care legislation requires that U.S. residents be implanted with RFID microchips.Claim: H.R. 4919, passed on 8 December 2016, allows the microchipping of "mentally disabled" citizens such as patients with autism and Alzheimer's disease. Many critics, including state legislators working to pass bills that would restrict RFID implants, are fearful that the metal components and circuitry in the chips would mean certain death if a.
Will 'Obamacare' Legislation Implant U.S. Residents with
Many US patients will have an implantable device during their lives. The AMA Code of Medical Ethics offers guidance for weighing need for patient-subjects’ safety against health care sector demand for innovation.
What Are the Benefits and Risks of Fitting Patients with
Since 1998, RFID chips have also been implanted in humans. This practice is little studied but appears to be increasing; rice-sized implants are implanted by hobbyists and even offered by some employers for uses ranging from access to emergency medical records to entry to secured workstations. Patients must trust that RFID devices will not be implanted or removed without their prior consent. When seeking patients' consent to implant an RFID device, physicians must do two things. First, they must disclose the possibility of unauthorized access to the information stored on the device.The implanted RFID devices enable patients to establish health care identities and become the stewards of their own data. The patient can assemble a reconciled medication list, a complete problem list, and a list of diagnostic study results, and then apply personal privacy preferences—for example, deleting information about mental health, HIV .This article reviews the use of implantable radiofrequency identification (RFID) tags in humans, focusing on the VeriChip (VeriChip Corporation, Delray Beach, FL) and the associated VeriMed patient identification system.
In the face of this emerging technology, it is essential that hand surgeons recognize the nuances of treating patients who have implanted RFID chips and also the promise and risk of this technology within the field of health care.
With an implanted RFID device, individuals can be tracked surreptitiously by anyone using a generic RFID reader, available for just a few hundred dollars. The informed consent process needs to.Claim: Health care legislation requires that U.S. residents be implanted with RFID microchips.
Claim: H.R. 4919, passed on 8 December 2016, allows the microchipping of "mentally disabled" citizens such as patients with autism and Alzheimer's disease.
Many critics, including state legislators working to pass bills that would restrict RFID implants, are fearful that the metal components and circuitry in the chips would mean certain death if a. Many US patients will have an implantable device during their lives. The AMA Code of Medical Ethics offers guidance for weighing need for patient-subjects’ safety against health care sector demand for innovation. Since 1998, RFID chips have also been implanted in humans. This practice is little studied but appears to be increasing; rice-sized implants are implanted by hobbyists and even offered by some employers for uses ranging from access to emergency medical records to entry to secured workstations.
Patients must trust that RFID devices will not be implanted or removed without their prior consent. When seeking patients' consent to implant an RFID device, physicians must do two things. First, they must disclose the possibility of unauthorized access to the information stored on the device.The implanted RFID devices enable patients to establish health care identities and become the stewards of their own data. The patient can assemble a reconciled medication list, a complete problem list, and a list of diagnostic study results, and then apply personal privacy preferences—for example, deleting information about mental health, HIV .
smart card certisign
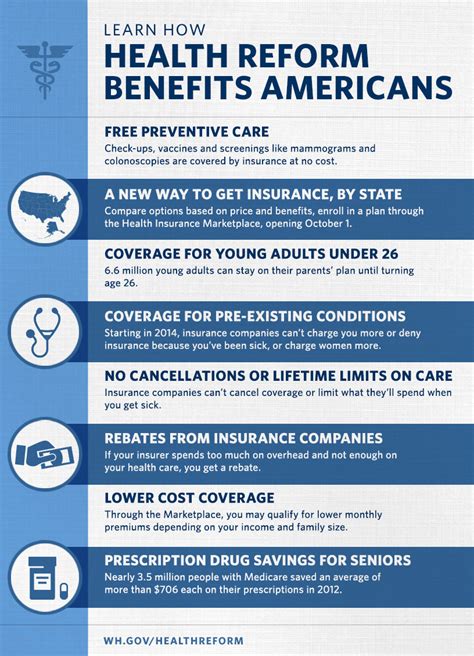
This article reviews the use of implantable radiofrequency identification (RFID) tags in humans, focusing on the VeriChip (VeriChip Corporation, Delray Beach, FL) and the associated VeriMed patient identification system.
smart card bus pass stagecoach
In the face of this emerging technology, it is essential that hand surgeons recognize the nuances of treating patients who have implanted RFID chips and also the promise and risk of this technology within the field of health care.
What Are the Benefits and Risks of Fitting Patients with
Please sign up to create a new account to manage your proofs and order status, .
implantable rfid chip in health care bill|Did Congress Pass a Bill Allowing the Government to Microchip